X chromosome inactivation is a fascinating biological process that plays a crucial role in the lives of females. This unique chromosomal mechanism ensures that one of the two X chromosomes in female mammals is silenced, preventing an overdose of gene dosage compared to males, who possess only one X chromosome. Understanding X chromosome inactivation has far-reaching implications, particularly in the study of genetic disorders such as Fragile X syndrome and Rett syndrome. Researchers, including Jeannie Lee and her team at Harvard Medical School, are uncovering the complex biophysical properties of DNA that underlie this process, potentially leading to advanced treatments for these conditions. With chromosomal research providing insights into gene silencing, the hope grows to develop effective Rett syndrome treatments and improve outcomes for those affected by genetic mutations on the X chromosome.
The phenomenon of X chromosome inactivation, often referred to as X-inactivation, is critical to female mammals, wherein one of the inherited X chromosomes is rendered inactive to balance gene expression with males. This process has significant implications in genetics, especially concerning various X-linked disorders such as Fragile X syndrome and Rett syndrome, which are influenced by mutations found on these chromosomes. Chromosomal studies focus on the intricate interactions that regulate gene silencing and contribute to our understanding of potential therapies. As researchers delve into the biophysical characteristics of DNA and the mechanisms behind chromosomal inactivation, the door opens to innovative treatments that could alter the course of these genetic disorders. The journey into this unique aspect of genetics may ultimately present viable Rett syndrome treatments through targeted manipulation of gene expression.
Understanding X Chromosome Inactivation
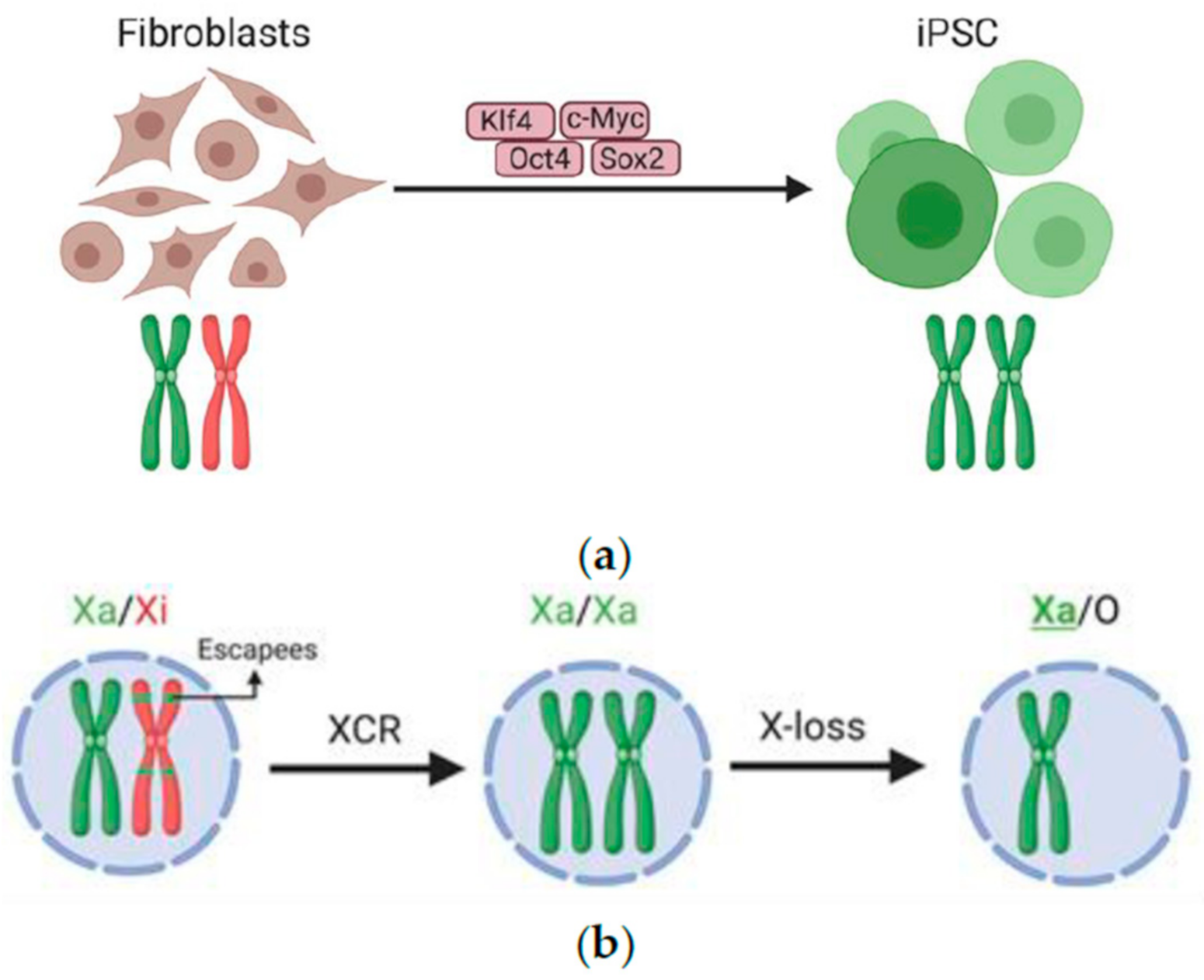
X chromosome inactivation (XCI) is a vital biological process that helps balance gene expression between males and females. Since females possess two X chromosomes while males carry one, this mechanism ensures that only one of the female’s Xs is active, preventing an overload of gene expression. The intricate process of XCI involves a series of molecular interactions whereby the gene Xist plays a crucial role. When Xist RNA translocates to the X chromosome, it triggers a cascade of changes that ultimately lead to the silencing of one X chromosome in female cells.
The significance of X chromosome inactivation extends beyond basic genetics to implications for understanding genetic disorders. Mutations found on the X chromosome can lead to various diseases, such as Rett syndrome and Fragile X syndrome, both of which are characterized by intellectual disabilities and developmental challenges. By further unraveling the mechanisms behind XCI, researchers like Jeannie Lee and her team aim to unlock potential treatments for these conditions, potentially mitigating their impact on affected individuals.
The mechanics of XCI involve a gelatinous matrix around chromosomes, likened to Jell-O, which helps in the organization and silencing of genes. This matrix aids in separating the chromosomes and is modified by RNA molecules like Xist. During XCI, Xist changes the physical properties of this matrix, allowing other proteins and molecules to infiltrate and silence the chromosome effectively. Understanding how these molecular forces interact not only provides insights into XCI but also into chromosomal research and its implications for treating X-linked genetic disorders.
As we delve deeper into XCI, it becomes apparent that this phenomenon can open doors to innovative therapies for various genetic disorders. The possibility of ‘unsilencing’ inactivated genes holds promise for individuals affected by conditions like Fragile X and Rett syndromes, where functional copies of genes are crucial. This therapeutic angle highlights why X chromosome biology is an exciting frontier in genetic research, showcasing the potential to restore normal cellular function through advanced medical approaches.
Frequently Asked Questions
What is X chromosome inactivation and why is it important for genetic disorders?
X chromosome inactivation is a biological process that occurs in female mammals where one of the two X chromosomes is randomly silenced to ensure dosage compensation. This mechanism is crucial in understanding genetic disorders such as Rett syndrome and Fragile X syndrome, as it helps prevent an overload of essential gene products and can impact how mutations on the X chromosome manifest.
How does X chromosome inactivation relate to treatments for Rett syndrome?
Research into X chromosome inactivation has opened potential avenues for treatments for Rett syndrome. By unsilencing the inactivated X chromosome, scientists aim to restore the expression of healthy genes that may be hindered by mutations, offering hope for therapeutic interventions.
Can X chromosome inactivation provide insights into Fragile X syndrome?
Yes, studies of X chromosome inactivation offer valuable insights into Fragile X syndrome, which is caused by mutations on the X chromosome. By understanding how X inactivation works, researchers are exploring ways to target and potentially reactivate the healthy gene copy that is silenced, which could lead to innovative treatments for those affected by the syndrome.
What role do biophysical properties of DNA play in X chromosome inactivation?
The biophysical properties of DNA are key to X chromosome inactivation. A gelatinous substance, compared to Jell-O, coats the X chromosome and changes its properties upon the binding of Xist RNA. This results in structural changes that contribute to the inactivation process, effectively silencing one of the two X chromosomes in females.
How does chromosomal research enhance our understanding of X chromosome inactivation?
Chromosomal research enhances our understanding of X chromosome inactivation by revealing the mechanisms through which cells can silence genes effectively. Studies led by researchers like Jeannie Lee have uncovered the ways that Xist interacts with chromosomal materials, which could lead to breakthroughs in treating genetic disorders linked to the X chromosome.
| Key Points | Details |
|---|---|
| X Chromosome Inactivation | Females have two X chromosomes; one must be inactivated to prevent gene dosage imbalance. |
| Role of Xist | The X chromosome produces Xist RNA, which interacts with chromosomal ‘Jell-O’ to facilitate inactivation. |
| Potential Treatments | Methods to unsilence genes on the inactive X chromosome could lead to therapies for Fragile X and Rett Syndromes. |
| Research Significance | The findings offer hope for treating conditions linked to mutations on the X chromosome. |
| Challenges Remaining | Understanding why only mutated genes become functional while healthy ones remain unaffected is still needed. |
Summary
X chromosome inactivation is a crucial biological process allowing females to balance gene expression from their two X chromosomes. Research led by Jeannie T. Lee has unveiled significant mechanisms behind this silencing, particularly the role of the RNA molecule Xist in modifying chromosomal structures. These insights not only enhance our understanding of cellular biology but also pave the way for innovative treatments for genetic disorders linked to the X chromosome, offering hope for individuals affected by Fragile X Syndrome and Rett Syndrome.