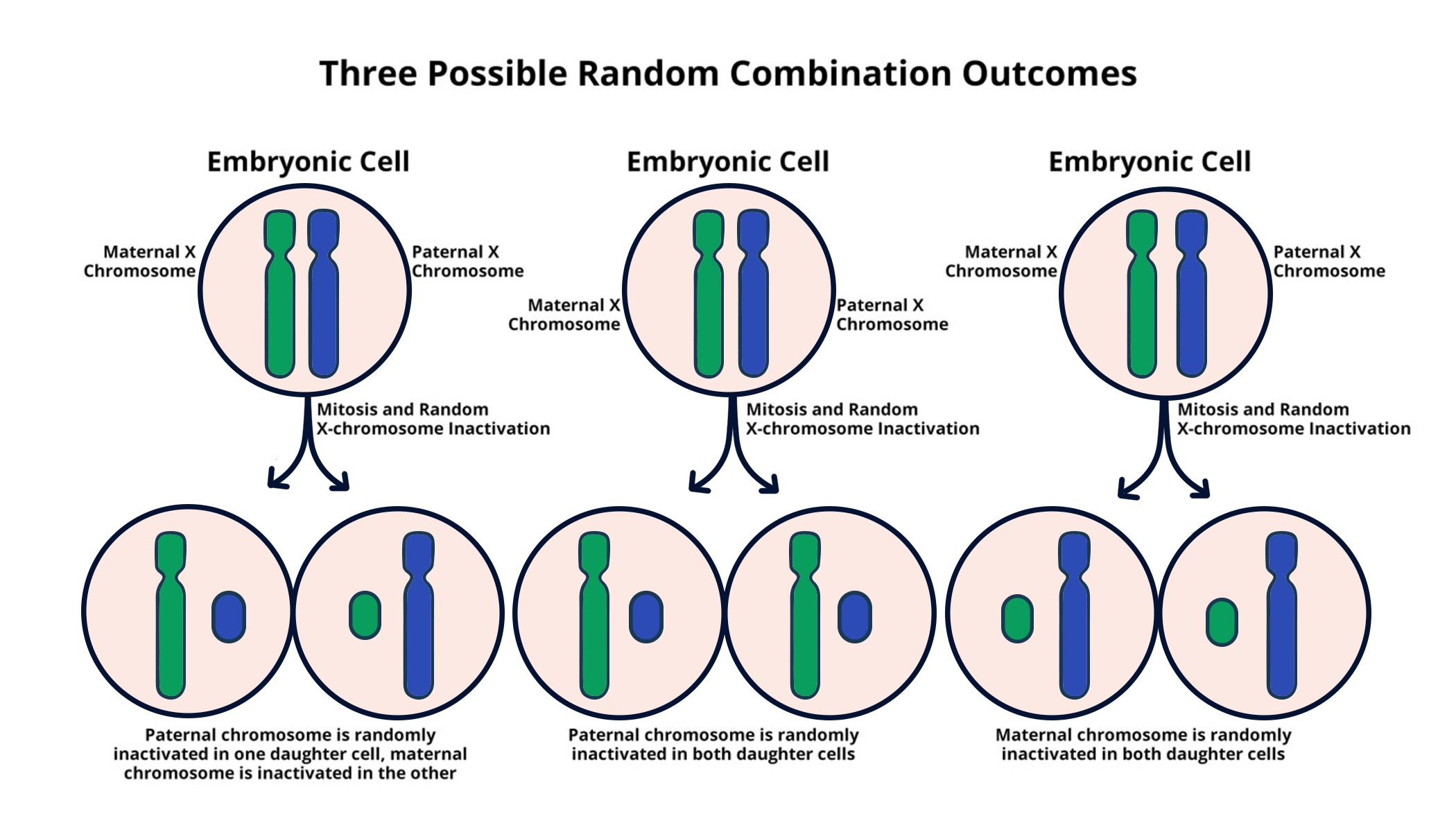
X chromosome inactivation is a fascinating process crucial to understanding genetic diseases linked to the X chromosome, such as Fragile X syndrome and Rett syndrome. In females, the presence of two X chromosomes necessitates the inactivation of one to maintain genetic balance, preventing an overabundance of genes. This complex mechanism involves an RNA molecule called Xist, which plays a significant role in silencing one of the X chromosomes, effectively acting as a switch that may lead to potential therapies. As researchers delve deeper into the implications of X chromosome inactivation, exciting possibilities for chromosomal therapy emerge. By uncovering the intricacies of this genetic phenomenon, scientists are opening doors to novel treatments that could reshape the lives of many affected by these chromosomal disorders.
Also referred to as X-inactivation, this biological process is essential in regulating gene expression in females by ensuring that only one of the two X chromosomes is active. The intricate mechanism is vital for preventing diseases associated with excess gene dosage, such as those seen in Fragile X syndrome and Rett syndrome. Research has shown that the Xist RNA is a key player in this silencing act, demonstrating the potential for innovative chromosomal therapy approaches. As scientists like Jeannie T. Lee explore the dynamics of X chromosome inactivation, they seek to unlock therapeutic strategies that could reverse the effects of genetic mutations found on these chromosomes. Understanding this phenomenon not only enhances knowledge of cellular function but also holds promise for new interventions in the field of genetics.
Understanding X Chromosome Inactivation
X chromosome inactivation (XCI) is a vital process that occurs in females to ensure that they do not express twice the number of genes coded on the X chromosome. This complex mechanism involves the random silencing of one of the two X chromosomes present in each cell early in embryonic development. The pioneering work conducted by Jeannie T. Lee’s lab has shed light on the intricate details of this process, elucidating how cells employ molecules like Xist RNA to mediate chromosomal silencing. This understanding is crucial, especially considering the implications for genetic diseases such as Fragile X syndrome, which is caused by mutations on the X chromosome. By effectively grasping how XCI operates, researchers are hopeful about devising innovative therapies to tackle such genetic conditions.
Furthermore, the significance of X chromosome inactivation is underscored by its role in various genetic diseases. For instance, more than 60% of genetic diseases associated with the X chromosome impact females, making XCI a crucial mechanism to explore in genetics research. The process of inactivation not only prevents gene dosage imbalances between sexes but also serves as a gateway to potential therapies aimed at reactivating mutated genes. The technology emerging from this area has implications that go beyond female disorders; even males, who typically have only one X chromosome, could benefit from treatments developed through insights gained about XCI. Understanding the nuances of this physiological process paves the way for chromosomal therapies that could drastically change the treatment landscape for countless patients.
The Role of Xist RNA in Chromosomal Therapy
Xist RNA is a pivotal molecule in the process of X chromosome inactivation (XCI) and has garnered much attention from researchers exploring potential therapies for genetic diseases. As cells undergo XCI, Xist RNA coats the designated X chromosome, altering the surrounding chromatin environment to achieve silencing. Jeannie Lee’s research highlights the fascinating dynamics between Xist RNA and the gelatinous material enveloping the chromosome, likening it to a ‘Jell-O’ that must be manipulated for effective gene regulation. This mechanism is a double-edged sword; while it serves to silence potentially harmful mutations, it also encapsulates the healthy gene variants that could be used for therapeutic purposes, especially in conditions like Fragile X syndrome and Rett syndrome. With advancements in our understanding of Xist RNA, the potential for targeted therapies becomes more attainable.
The therapeutic applications of Xist-derived strategies are particularly exciting for developing chromosomal therapies. By leveraging the inherent properties of Xist RNA, researchers can envision ways to reactivate silenced genes that may lead to functional recovery in affected individuals. As shown in Lee’s laboratory experiments, reversing XCI has shown promise in preliminary studies, suggesting that we might be able to restore the function of genes responsible for conditions linked to the X chromosome. Moreover, the prospect of selectively targeting specific genes without impacting other healthy variants represents a monumental shift in the treatment paradigm for genetic diseases. If successful, these approaches could lead to innovative therapies that effectively mitigate the genetic burden posed by disorders such as Fragile X syndrome and Rett syndrome.
Implications for Genetic Diseases and Future Treatments
The implications of X chromosome inactivity and the associated research conducted by Lee and her team extend far beyond theoretical considerations; they pave the way for promising future treatments. Diseases like Fragile X syndrome and Rett syndrome are characterized by mutation-driven gene dysfunctions, often caused by aberrations on the X chromosome. By potentially reactivating the silenced genes on this chromosome, researchers hope to provide therapeutic avenues that could lead to improved outcomes for individuals grappling with these genetic disorders. This brute-force approach toward gene therapy could allow patients to leverage the healthy gene expression that is otherwise dormant in their cells due to XCI.
Additionally, the implications of understanding X chromosome inactivation reach into the realm of personalized medicine. With growing understanding around techniques to manipulate and study XCI, there exists a golden opportunity to individualize treatment strategies based on the specific genetic makeup of patients. Chromosomal therapy does not only aim to tackle genetic diseases but also strives for minimal side effects by ensuring that healthy genetic mechanisms remain untouched while dysfunctional genes are targeted. As this field of genetic therapy evolves, ongoing research into the details of chromosomal inactivity will be crucial in advancing innovative strategies against diseases like Fragile X and Rett syndrome, potentially transforming the lives of millions affected globally.
Advancements in Chromosomal Therapy
Recent advancements in chromosomal therapy have positioned scientists on the brink of groundbreaking treatments for genetic diseases linked with the X chromosome. These therapies stem from crucial insights gained through extensive research on X chromosome inactivation (XCI) and its molecular underpinnings, particularly the role of Xist RNA. As scientists improve their understanding of how XCI is achieved, they are also developing techniques that could effectively reverse this process, unearthing new possibilities for restoring gene function. With the ability to reactivate previously inactive genes, there is the potential to provide much-needed relief for patients suffering from disorders like Fragile X syndrome.
Moreover, these advancements hold the promise not just for female patients but also for males who might harbor mutations that result in conditions typically associated with X-linked genes. The therapeutic potential of chromosomal therapy lies in its ability to fine-tune gene regulation without unintended consequences—a major concern in traditional gene therapy. Researchers are optimistic that with the right approaches, including genomic editing technologies complemented by insights from XCI studies, the next generation of treatments could lead to durable and effective cures with minimal adverse effects on healthy gene expression.
Research Funding and Future Directions
The research accomplishments surrounding X chromosome inactivation and chromosomal therapy are not merely academic; they have been supported by substantial funding from reputable sources, including the National Institutes of Health. This backing reflects the significance of research endeavors that bridge the gap between fundamental biology and practical medicine. The insights offered by Jeannie T. Lee’s team have not only elucidated critical aspects of XCI but also brought forth potential therapeutic pathways for debilitating genetic diseases, such as Fragile X syndrome and Rett syndrome. As research continues to unfold, the sustained financial and academic support is crucial in ensuring these findings translate into effective clinical applications.
Looking towards the future, the continued exploration of X-linked genetic diseases and the development of chromosomal therapies are essential steps in the fight against genetic disorders. The emphasis on understanding how XCI operates, coupled with the innovative use of molecular biology techniques, could enable breakthroughs that were previously deemed unattainable. By effectively employing targeted therapies, medical science stands at the frontier of potentially curing conditions that have long been associated with genetic predispositions, radically altering the landscape of genetic disease management.
The Influence of Genetic Disorders on X Chromosome Inactivation
The complexity of genetic disorders linked to the X chromosome significantly influences the understanding and mechanisms of X chromosome inactivation (XCI). Conditions such as Fragile X syndrome and Rett syndrome illustrate how mutations can disrupt normal gene functioning, leading to profound intellectual and developmental challenges. The necessity of XCI in females ensures gene dosage balance; however, when mutations exist on one X chromosome, the need for reactivation becomes crucial in restoring health. Insights from research have revealed how Xist RNA not only plays a significant role in initiating XCI but can also be manipulated to potentially reactivate affected genes. This relationship between genetic disorders and XCI lays the groundwork for developing novel therapies aimed at rectifying the adverse effects of mutations.
Additionally, the mechanisms underlying X chromosome inactivation shed light on potential therapeutic interventions for these genetic conditions. The interplay between cellular regulation and genetic expression forms the backbone of strategies aimed at treating disorders like Fragile X syndrome, which is characterized by excessive repeats in the FMR1 gene. Understanding how XCI can be effectively reversed opens avenues for developing therapies that can target and silence harmful mutant alleles while preserving the function of healthy genes. As our comprehension deepens, so too does the potential for innovative treatments that could alleviate the burden of X-linked genetic diseases and improve patient outcomes.
Challenges in Reactivating Inactivated Genes
Reactivating inactivated genes presents numerous challenges that researchers must navigate to develop effective therapies for X-linked disorders. While the concept of reversing X chromosome inactivation (XCI) to restore gene function is promising, the multifaceted nature of gene expression regulation poses significant obstacles. One such challenge lies in identifying the precise mechanisms by which Xist RNA exerts its influence on chromatin structure and gene silencing. Moreover, ensuring that the reactivation process selectively targets only the defective genes without affecting the silent but functional copies remains a complex endeavor. The understanding of these intricacies is essential for scientists to harness the power of chromosomal therapy effectively.
Moreover, the safety and efficacy of reactivating inactivated genes require thorough investigation before clinical application. Researchers must consider the potential risk of unintended consequences in restoring gene function, such as the activation of harmful mutations or disruption of other necessary gene pathways. Balancing these factors will determine the feasibility of translating laboratory findings into real-world treatments for genetic diseases associated with the X chromosome. Despite these challenges, the potential benefits of successfully reactivating inactivated genes could pave the way for revolutionary therapies that transform the landscape for individuals affected by X-linked genetic disorders.
Ethical Considerations in Genetic Therapies
As with any rapidly advancing field, ethical considerations arise when exploring therapies aimed at reactivating inactivated genes on the X chromosome. The implications of altering gene expression, particularly through chromosomal therapies, raise significant questions concerning consent, equity in access to treatments, and potential long-term effects on patients. Ethical discussions must include the potential for genetic therapies to unintentionally initiate unintended biological consequences, alongside the risks inherent in using experimental techniques such as gene editing. Engaging with these ethical dilemmas is critical as the field progresses, ensuring that the benefits of such therapies can be responsibly and equitably shared across diverse populations.
Moreover, considerations of equity in access highlight the disparities that may arise in the application of advanced genetic therapies. As research transitions from laboratory to clinic, the cost and availability of such treatments can create barriers that disproportionately affect specific communities. This highlights the need for inclusive discussions involving scientists, ethicists, and policymakers to ensure that breakthroughs in treatments derived from understanding X chromosome inactivation are accessible to all those affected by genetic disorders. These deliberations form the cornerstone of responsible scientific development and advocacy in addressing the challenges and promises posed by novel chromosomal therapies.
Frequently Asked Questions
What is X chromosome inactivation and why is it important for genetic diseases?
X chromosome inactivation (XCI) is a biological process by which one of the two copies of the X chromosome present in female mammals is inactivated to balance gene dosage with males, who have only one X chromosome. This process is crucial for understanding genetic diseases like Fragile X syndrome and Rett syndrome, as mutations affecting genes on the X chromosome can lead to these disorders. Proper knowledge of XCI could lead to the development of therapies aimed at reactivating inactivated X chromosomes to restore normal gene function.
How does Xist RNA play a role in X chromosome inactivation?
Xist RNA is a pivotal molecule in the process of X chromosome inactivation. It is produced by a gene on the X chromosome and coats the chromosome, leading to its silencing. The action of Xist changes the biophysical properties of the surrounding chromatin (described as ‘Jell-O’), making it conducive for gene inactivation. Research into Xist RNA provides insights into potential treatments for genetic diseases linked to mutations on the X chromosome, such as Fragile X syndrome and Rett syndrome.
How might understanding X chromosome inactivation help treat Fragile X syndrome?
Understanding X chromosome inactivation (XCI) may allow scientists to reactivate the healthy copy of the gene responsible for Fragile X syndrome that is typically silenced. By developing techniques to reverse the silencing of affected genes, researchers like Jeannie Lee propose that it may be possible to treat or even cure this genetic disorder. Insights into XCI and the role of molecules like Xist RNA are essential for advancing toward effective therapies.
What are the implications of X chromosome inactivation for male patients with genetic diseases?
Although males do not undergo X chromosome inactivation due to having only one X chromosome, understanding this process has significant implications for treating conditions like Fragile X syndrome. For males with mutations on their X chromosome, targeted therapies could selectively silence or activate genes to restore their function. The principles learned from XCI research can inform innovative treatments for male patients suffering from genetic diseases linked to X chromosome mutations.
What are the potential next steps in X chromosome inactivation research related to chromosomal therapy?
The next steps in X chromosome inactivation research involve optimizing techniques for reactivating silenced X-linked genes in affected individuals and conducting safety studies. Researchers aim to move promising therapies into clinical trials, especially those that could benefit conditions such as Fragile X syndrome and Rett syndrome. Continuous exploration of the mechanisms behind XCI will likely contribute to the development of effective chromosomal therapies.
| Key Point | Details |
|---|---|
| X Chromosome Inactivation | In females, one of the two X chromosomes is inactivated to ensure gene dosage balance with males who have one X. |
| Role of Xist RNA | Xist RNA modifies the surrounding chromosomal environment (gel-like material) making the X chromosome inactive. |
| Potential Therapies | Reactivating the inactivated X chromosome may offer new treatment options for genetic disorders such as Fragile X and Rett syndromes. |
| Ongoing Research | Lee’s lab is developing methods to reverse X-linked gene silencing in cells, targeting specific genetic disorders. |
| Clinical Implications | Research funding from NIH has led to promising findings that could translate into clinical trials. |
Summary
X chromosome inactivation is a crucial biological process that ensures appropriate gene dosage between males and females by silencing one of the two X chromosomes in females. Recent advancements in understanding how this inactivation occurs, particularly through the role of the Xist RNA molecule, reveal potential pathways for innovative therapies aimed at treating genetic diseases linked to the X chromosome. As researchers like Jeannie T. Lee clarify the mechanisms behind X chromosome inactivation, the possibility of reactivating an inactivated X holds the promise of therapeutic interventions for conditions such as Fragile X syndrome and Rett syndrome, offering hope to many affected individuals.